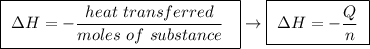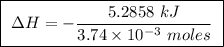Chemistry, 22.07.2019 18:00 ttwright24
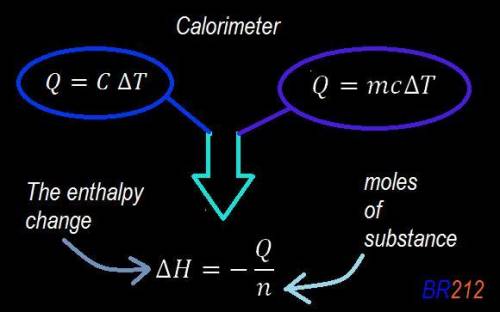
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.105-g sample of ethylene (c2h4) was burned in this calorimeter, the temperature increased by 2.14 k. calculate the energy of combustion for one mole of ethylene. a. –0.259 kj/mol b. –50.3 kj/mol c. –5.29 kj/mol d. –1.41 × 103 kj/mol e. –660 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2
You know the right answer?
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.105-g sample of ethylene (c2h4) was bur...
Questions


Computers and Technology, 22.10.2020 21:01

Mathematics, 22.10.2020 21:01


Biology, 22.10.2020 21:01

Chemistry, 22.10.2020 21:01

Mathematics, 22.10.2020 21:01


German, 22.10.2020 21:01

Mathematics, 22.10.2020 21:01

English, 22.10.2020 21:01

Advanced Placement (AP), 22.10.2020 21:01

Advanced Placement (AP), 22.10.2020 21:01

Advanced Placement (AP), 22.10.2020 21:01

Mathematics, 22.10.2020 21:01





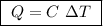
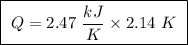
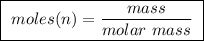
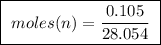
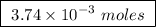 of ethylene.
of ethylene.