

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 2

You know the right answer?
Tin (sn) exists in earth's crust as sno2. calculate the percent composition by mass of sn and o in s...
Questions

Mathematics, 27.06.2019 21:30



Mathematics, 27.06.2019 21:30

Mathematics, 27.06.2019 21:30

Biology, 27.06.2019 21:30


Physics, 27.06.2019 21:30





Mathematics, 27.06.2019 21:30

English, 27.06.2019 21:30






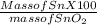
 %.
%.

