
Chemistry, 18.07.2019 05:50 davidsouth444
Consider the combustion reaction for acetylene. 2c2h2(l) + 5o2(g) mc001-1.jpg 4co2(g) + 2h2o(g) if the acetylene tank contains 37.0 mol of c2h2 and the oxygen tank contains 81.0 mol of o2, what is the limiting reactant for this reaction?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1


Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
Consider the combustion reaction for acetylene. 2c2h2(l) + 5o2(g) mc001-1.jpg 4co2(g) + 2h2o(g) if t...
Questions

Computers and Technology, 17.12.2021 01:40











Mathematics, 17.12.2021 01:40

Mathematics, 17.12.2021 01:40




Mathematics, 17.12.2021 01:40



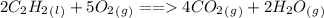
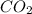 by each reactant.
by each reactant.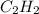
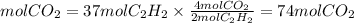
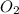
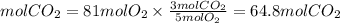
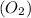 will be finished when 64.8mol of
will be finished when 64.8mol of 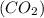 have been produced. There will be excess acetylene
have been produced. There will be excess acetylene 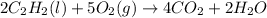
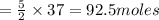 of oxygen.
of oxygen.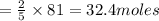 of acetylene.
of acetylene.


