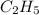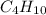Chemistry, 18.07.2019 00:00 hihowareyou12
Asample of a hydrocarbon contains 20.75 g c and 4.25 g h . it’s molar mass is 58.04 g/mol . what is its empirical formula

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
You know the right answer?
Asample of a hydrocarbon contains 20.75 g c and 4.25 g h . it’s molar mass is 58.04 g/mol . what is...
Questions



English, 12.09.2021 05:30

History, 12.09.2021 05:30

Business, 12.09.2021 05:30

Mathematics, 12.09.2021 05:30

Physics, 12.09.2021 05:30

Mathematics, 12.09.2021 05:30


Mathematics, 12.09.2021 05:30

Spanish, 12.09.2021 05:30


History, 12.09.2021 05:30

Mathematics, 12.09.2021 05:30

Mathematics, 12.09.2021 05:30

Mathematics, 12.09.2021 05:30

Mathematics, 12.09.2021 05:30