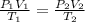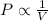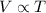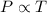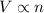Chemistry, 17.07.2019 12:50 nightwolf8999
The table below shows a comparison of the different gas laws. some cells have been left blank. name variables constants equation boyle's law pressure, volume ? pv = k charles’s law volume, temperature ? v = kt ? temperature, pressure volume, moles of gas p = kt ? pressure, temperature, volume ? mc022-1.jpg which are assumed to be constant while using the combined gas law? a. pressure b. number of moles c. volume and moles of gas d. pressure and temperature

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3

Chemistry, 23.06.2019 09:30
What is the force of an object when it landed(sitting in the ground)
Answers: 2

You know the right answer?
The table below shows a comparison of the different gas laws. some cells have been left blank. name...
Questions

Mathematics, 18.11.2019 01:31


History, 18.11.2019 01:31

Mathematics, 18.11.2019 01:31

Mathematics, 18.11.2019 01:31

Mathematics, 18.11.2019 01:31


History, 18.11.2019 01:31

Chemistry, 18.11.2019 01:31

English, 18.11.2019 01:31


Physics, 18.11.2019 01:31

History, 18.11.2019 01:31


History, 18.11.2019 01:31


Business, 18.11.2019 01:31



Mathematics, 18.11.2019 01:31