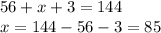Chemistry, 17.07.2019 08:00 rosebelll4555
After the fission of u-235 takes place, how many neutrons does the missing nucleus have? a) 84 b) 85 c) 141 d) 143 the atomic number is consider a unique atom and element identifier. describe the associated subatomic particle, and how it can be used to identify the atom. a) the number of electrons changes in a unique way as the charge on the atom changes. b) the proton number remains constant through changing the atomic charge, energy, and isotopes. c) as the atom absorbs photons the way the photons interact in the nucleus gives the atomic number. d) all isotopes of the an element have the same number of neutrons allowing it to describe the unique atom.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
You know the right answer?
After the fission of u-235 takes place, how many neutrons does the missing nucleus have? a) 84 b)...
Questions



Mathematics, 20.01.2021 22:10

Mathematics, 20.01.2021 22:10




Mathematics, 20.01.2021 22:10

Mathematics, 20.01.2021 22:10

Mathematics, 20.01.2021 22:10



History, 20.01.2021 22:10


Mathematics, 20.01.2021 22:10

Mathematics, 20.01.2021 22:10

English, 20.01.2021 22:10


Mathematics, 20.01.2021 22:10