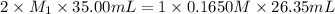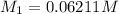Chemistry, 16.07.2019 03:40 rwlockwood1
If 26.35 ml of a standard 0.1650 m naoh solution is required to neutralize 35.00 ml of h2so4, what is the molarity of the acid solution?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:50
Your roll: experienced electron speech is adressed to: a new "freshman class" of electrons job: write a speech task: you are to pretend that you are giving a speech to a new group of electrons. be sure to mention their placement in an atom, their charge, and their role in chemical bonding (ionic and covalent) be specific!
Answers: 3

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
If 26.35 ml of a standard 0.1650 m naoh solution is required to neutralize 35.00 ml of h2so4, what i...
Questions

Physics, 13.10.2019 20:50


Mathematics, 13.10.2019 20:50

English, 13.10.2019 20:50


Mathematics, 13.10.2019 20:50

History, 13.10.2019 20:50


Mathematics, 13.10.2019 20:50


English, 13.10.2019 20:50



Health, 13.10.2019 20:50


Geography, 13.10.2019 20:50

Mathematics, 13.10.2019 20:50



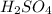 is, 0.06211 M
is, 0.06211 M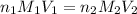
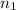 = basicity of an acid
= basicity of an acid 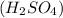 = 2
= 2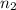 = acidity of a base
= acidity of a base 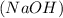 = 1
= 1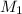 = concentration or molarity of
= concentration or molarity of 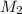 = concentration of NaOH = 0.1650 M
= concentration of NaOH = 0.1650 M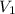 = volume of
= volume of 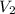 = volume of NaOH = 26.35 mL
= volume of NaOH = 26.35 mL