Chemistry, 16.07.2019 02:40 shelbymelton18
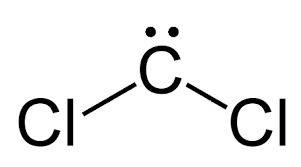
Acompound composed of only carbon and chlorine is 85.5% chlorine by mass. propose a lewis structure for the lightest of the possible compounds that allows each atom to have a complete octet without formal charges. draw the molecule by placing atoms on the grid and connecting them with bonds. include all lone pairs of electrons.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3

Chemistry, 23.06.2019 08:30
Of element x has 22 protons, how many electrons does it have
Answers: 1
You know the right answer?
Acompound composed of only carbon and chlorine is 85.5% chlorine by mass. propose a lewis structure...
Questions



Mathematics, 26.10.2020 02:30



Spanish, 26.10.2020 02:30

German, 26.10.2020 02:30


Mathematics, 26.10.2020 02:30

Mathematics, 26.10.2020 02:40

Mathematics, 26.10.2020 02:40

History, 26.10.2020 02:40


Mathematics, 26.10.2020 02:40


English, 26.10.2020 02:40

Mathematics, 26.10.2020 02:40

Mathematics, 26.10.2020 02:40


Mathematics, 26.10.2020 02:40