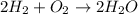Chemistry, 15.07.2019 17:40 brooklyn674
Read the scenario. two molecules of hydrogen gas and one molecule of oxygen gas react to yield two molecules of water. which of the following equations properly models the reaction described? h2 + o2 → h2o2 h2 + o2 → 2h2o h + o → h2o 2h2 + o2 → 2h2o

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
Read the scenario. two molecules of hydrogen gas and one molecule of oxygen gas react to yield two...
Questions

History, 11.03.2020 21:14






Health, 11.03.2020 21:15

History, 11.03.2020 21:15


History, 11.03.2020 21:15

Biology, 11.03.2020 21:15


Mathematics, 11.03.2020 21:16



History, 11.03.2020 21:16


Mathematics, 11.03.2020 21:16