

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2


Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3
You know the right answer?
A0.8838-g sample of an ionic compound containing bromide ions and an unknown metal cation is dissolv...
Questions




Mathematics, 17.03.2020 03:05

English, 17.03.2020 03:05







Social Studies, 17.03.2020 03:06

Computers and Technology, 17.03.2020 03:06




Computers and Technology, 17.03.2020 03:06



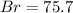 %
%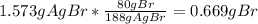
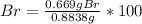 %
%

