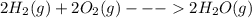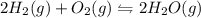Consider the chemical equation in equilibrium. 2h2(g) + o2(g) mc007-1.jpg 2h2o(g) what will happen if the pressure of the system is increased? the reaction will not change. the reverse reaction will be favored. the reaction will stop completely. the forward reaction will be favored.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2
You know the right answer?
Consider the chemical equation in equilibrium. 2h2(g) + o2(g) mc007-1.jpg 2h2o(g) what will happen...
Questions



Physics, 15.07.2021 18:50


Mathematics, 15.07.2021 18:50

Mathematics, 15.07.2021 19:00

English, 15.07.2021 19:00



Mathematics, 15.07.2021 19:00

English, 15.07.2021 19:00

Mathematics, 15.07.2021 19:00

English, 15.07.2021 19:00

English, 15.07.2021 19:00

Biology, 15.07.2021 19:00

Mathematics, 15.07.2021 19:00

Social Studies, 15.07.2021 19:00

Social Studies, 15.07.2021 19:00

Social Studies, 15.07.2021 19:00

Mathematics, 15.07.2021 19:00