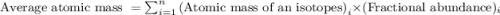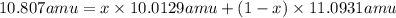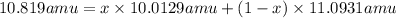Chemistry, 14.07.2019 02:50 mlarsen5000
The average atomic masses of some elements may vary, depending upon the sources of their ores. naturally occurring boron consists of two isotopes with accurately known masses (10b, 10.0129 amu and 11b, 11.0931 amu). the actual atomic mass of boron can vary from 10.807 to 10.819, depending on whether the mineral source is from turkey or the united states. calculate the percent abundances leading to the two values of the average atomic masses of boron from these two countries.

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
The volume of a sphere is given by v= (4/3) pi r cubed, where r is the radius. compute the volume of a sphere with a radius of 117pm. state your answer in units of cubed.
Answers: 1

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 22.06.2019 00:10
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3
You know the right answer?
The average atomic masses of some elements may vary, depending upon the sources of their ores. natur...
Questions

English, 11.03.2021 02:50

History, 11.03.2021 02:50

Physics, 11.03.2021 02:50

Mathematics, 11.03.2021 02:50



Mathematics, 11.03.2021 02:50


Mathematics, 11.03.2021 02:50

Mathematics, 11.03.2021 02:50

English, 11.03.2021 02:50

History, 11.03.2021 02:50

Social Studies, 11.03.2021 02:50

Mathematics, 11.03.2021 02:50

History, 11.03.2021 02:50


Mathematics, 11.03.2021 02:50

Mathematics, 11.03.2021 02:50


Spanish, 11.03.2021 02:50