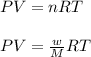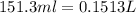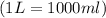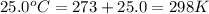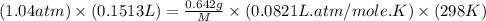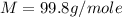Chemistry, 13.07.2019 13:30 lizdominguez101
A0.642g sample of an unknown gas was collected over water at 25.0°c and 1.04 atm. the collection cylinder contained 151.3 ml of gas after the sample was released. find the molar mass of the unknown gas.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 23.06.2019 00:00
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1
You know the right answer?
A0.642g sample of an unknown gas was collected over water at 25.0°c and 1.04 atm. the collection cyl...
Questions






Mathematics, 05.09.2020 09:01






Mathematics, 05.09.2020 09:01


Biology, 05.09.2020 09:01

Mathematics, 05.09.2020 09:01





Computers and Technology, 05.09.2020 09:01