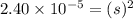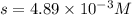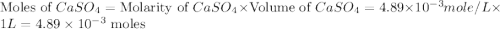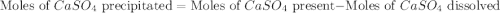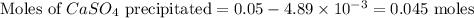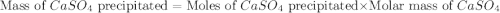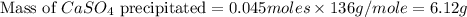Chemistry, 13.07.2019 11:00 gildedav001
If 500.0 ml of 0.10 m ca2+ is mixed with 500.0 ml of 0.10 m so42−, what mass of calcium sulfate will precipitate? ksp for caso4 is 2.40×10−5. express your answer to three significant figures and include the appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1
You know the right answer?
If 500.0 ml of 0.10 m ca2+ is mixed with 500.0 ml of 0.10 m so42−, what mass of calcium sulfate will...
Questions

Mathematics, 11.09.2021 03:50

Computers and Technology, 11.09.2021 03:50

Mathematics, 11.09.2021 03:50

Mathematics, 11.09.2021 03:50



Mathematics, 11.09.2021 04:00



Mathematics, 11.09.2021 04:00

Mathematics, 11.09.2021 04:00

Chemistry, 11.09.2021 04:00

Engineering, 11.09.2021 04:00


Mathematics, 11.09.2021 04:00


Mathematics, 11.09.2021 04:00



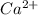 and
and 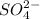 .
.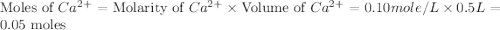
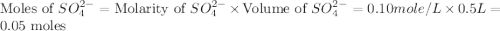
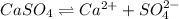
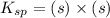
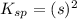
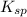 in this expression, we get the solubility of calcium sulfate.
in this expression, we get the solubility of calcium sulfate.