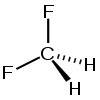
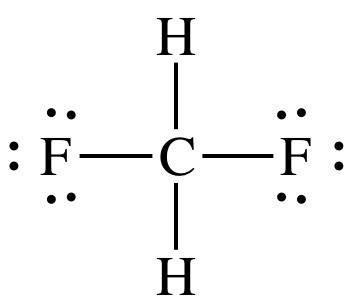
Problem 9.4 the molecule shown here is difluoromethane (ch2f2), which is used as a refrigerant called r-32. a carbon is single bonded left and right, angled above, to f, and below, angled in and out of the page , to h. part a based on the structure, how many electron domains surround the c atom in this molecule? express your answer as an integer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Problem 9.4 the molecule shown here is difluoromethane (ch2f2), which is used as a refrigerant calle...
Questions


Computers and Technology, 16.02.2021 18:10


English, 16.02.2021 18:10

Social Studies, 16.02.2021 18:10






Mathematics, 16.02.2021 18:10

Mathematics, 16.02.2021 18:10

Mathematics, 16.02.2021 18:10

Mathematics, 16.02.2021 18:10


Chemistry, 16.02.2021 18:10

English, 16.02.2021 18:10



Chemistry, 16.02.2021 18:10