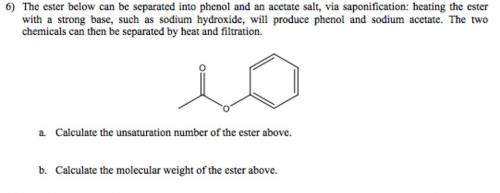
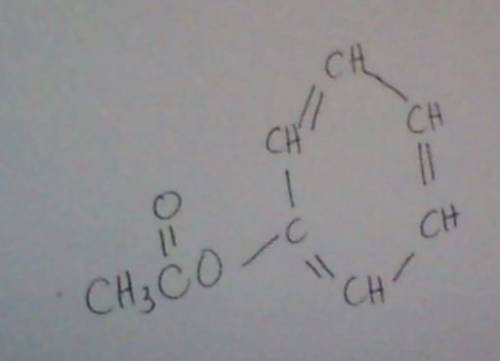
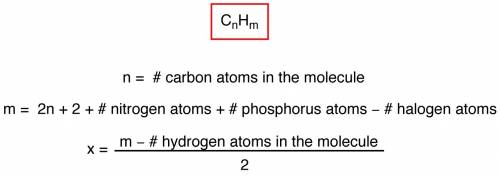
The ester below can be separated into phenol and an acetate salt, via saponification: heating the ester with a strong base, such as sodium hydroxide, will produce phenol and sodium acetate. the two chemicals can then be separated by heat and filtration. a. calculate the unsaturation number of the ester above. b. calculate the molecular weight of the ester above

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 23.06.2019 04:40
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2

Chemistry, 23.06.2019 06:00
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2
You know the right answer?
The ester below can be separated into phenol and an acetate salt, via saponification: heating the e...
Questions


English, 12.11.2020 22:50


Mathematics, 12.11.2020 22:50

World Languages, 12.11.2020 22:50



Mathematics, 12.11.2020 22:50

Geography, 12.11.2020 22:50



Mathematics, 12.11.2020 22:50


Biology, 12.11.2020 22:50


Social Studies, 12.11.2020 22:50



Mathematics, 12.11.2020 22:50