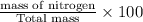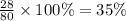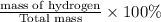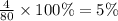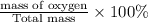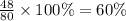The last step is to calculate the percent by mass of each element in ammonium nitrate (nh4no3). the masses of the elements in one mole of the compound are: mass n = 28.0 g mass h = 4.0 g mass o = 48.0 g the molar mass of the compound is 80.0 g/mol. what is the mass of one mole of the compound?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
The last step is to calculate the percent by mass of each element in ammonium nitrate (nh4no3). the...
Questions

Advanced Placement (AP), 24.02.2021 22:40



English, 24.02.2021 22:40


History, 24.02.2021 22:40




Social Studies, 24.02.2021 22:40

Mathematics, 24.02.2021 22:40


Physics, 24.02.2021 22:40


Mathematics, 24.02.2021 22:40


Chemistry, 24.02.2021 22:40

Social Studies, 24.02.2021 22:40

Biology, 24.02.2021 22:40

Mathematics, 24.02.2021 22:40