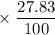Chemistry, 10.07.2019 20:50 shekinahdavis8760
Calculate the average atomic mass of rubidium. rubidium has two isotopes, 85rb and 87rb. 85rb has an atomic mass of 84.912 amu and occurs at an abundance of 72.17%. 87rb has an atomic mass of 86.909 amu and occurs at an abundance of 27.83%. show your work.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 23.06.2019 10:00
An uncovered pot of water lies out in the sun. which statements correctly describe what happens at the surface of the liquid water? 1. the vapor pressure remains constant regardless of the water temperature. 2. the vapor pressure is produced by water molecules that have evaporated. 3. the vapor pressure increases as the sun heats the water in the pot. 4. evaporation stops once the vapor pressure reaches a certain point. 5. evaporation and condensation both occur on the liquid’s surface.
Answers: 3

Chemistry, 23.06.2019 12:00
Explaining why atoms bondcomplete the sentence.atoms form chemical bonds to satisfy the rule and to become .
Answers: 1
You know the right answer?
Calculate the average atomic mass of rubidium. rubidium has two isotopes, 85rb and 87rb. 85rb has an...
Questions


Mathematics, 06.04.2020 11:20

Mathematics, 06.04.2020 11:20

History, 06.04.2020 11:20

Mathematics, 06.04.2020 11:21


Mathematics, 06.04.2020 11:21

Mathematics, 06.04.2020 11:21

Mathematics, 06.04.2020 11:21

Business, 06.04.2020 11:21

English, 06.04.2020 11:21


Mathematics, 06.04.2020 11:21

Mathematics, 06.04.2020 11:21


English, 06.04.2020 11:21

Mathematics, 06.04.2020 11:22

Mathematics, 06.04.2020 11:22


Mathematics, 06.04.2020 11:22

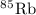 has atomic mass = 84.912 amu
has atomic mass = 84.912 amu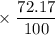
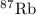 has atomic mass = 86.909 amu
has atomic mass = 86.909 amu