
Chemistry, 31.08.2019 16:30 theyfallfora
Larutan kcl dengan konsentrasi 0,5 m dapat dibuat dengan melarutkan serbuk kcl ke dalam 100 ml air. jika diketahui mr kcl 74,5 massa kcl yang harus dilarutkan adalah

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Problem #3 (ch. 1, problem 15)the ideal gas law provides one way to estimate the pressure exerted by a gas on a container. the law isí‘ťí‘ť=푛푛푛푛푛푛푉푉more accurate estimates can be made with the van der waals equationí‘ťí‘ť=푛푛푛푛푛푛푉푉â’푛푛푟푟â’푞푞푛푛2푉푉2where the term nb is a correction for the volume of the molecules and the term an2/v2is a correction for molecular attractions. the values of a and b depend on the type of gas. the gas constant is r, the absolutetemperature is t, the gas volume is v, and the number of moles of gas molecules is indicated by n. if n = 1 mol of an ideal gas were confined to a volume of v = 22.41 l at a temperature of 0â°c (273.2k), it would exert a pressure of 1 atm. in these units, r = 0.0826.for chlorine gas (cl2), a = 6.49 and b = 0.0562. compare the pressure estimates given by the ideal gas law and the van der waals equation for 1 mol of cl2 in 22.41 l at 273.2 k. what is the main cause of the difference in the two pressure estimates, the molecular volume or the molecular attractions?
Answers: 1

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2
You know the right answer?
Larutan kcl dengan konsentrasi 0,5 m dapat dibuat dengan melarutkan serbuk kcl ke dalam 100 ml air....
Questions

English, 04.01.2021 20:40


English, 04.01.2021 20:40

Biology, 04.01.2021 20:40

Computers and Technology, 04.01.2021 20:40





Chemistry, 04.01.2021 20:40

Mathematics, 04.01.2021 20:40


Mathematics, 04.01.2021 20:40

Mathematics, 04.01.2021 20:40


Geography, 04.01.2021 20:40


English, 04.01.2021 20:40

History, 04.01.2021 20:40

Mathematics, 04.01.2021 20:40

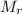 of KCl is 74.5, the mass should be _____.
of KCl is 74.5, the mass should be _____.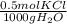 = 0.05 mol KCl
= 0.05 mol KCl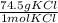 = 3.725 g KCl
= 3.725 g KCl

