
Chemistry, 10.07.2019 10:40 taysomoneyyy
Combustion of 25.0 g of a hydrocarbon produces 86.5 g of co2. what is the empirical formula of the compound?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
Combustion of 25.0 g of a hydrocarbon produces 86.5 g of co2. what is the empirical formula of the c...
Questions

Health, 08.12.2020 01:50



Mathematics, 08.12.2020 01:50

Spanish, 08.12.2020 01:50

Mathematics, 08.12.2020 01:50

Mathematics, 08.12.2020 01:50

English, 08.12.2020 01:50


Mathematics, 08.12.2020 01:50


Mathematics, 08.12.2020 01:50

Mathematics, 08.12.2020 01:50




Mathematics, 08.12.2020 01:50

Mathematics, 08.12.2020 01:50

Mathematics, 08.12.2020 01:50

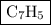 .
.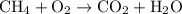
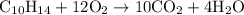
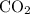 is formed as a product during combustion reactions.
is formed as a product during combustion reactions.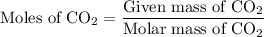 ...... (1)
...... (1)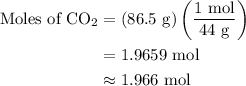
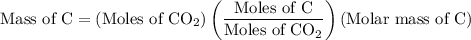 ...... (2)
...... (2)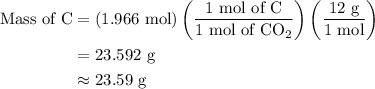
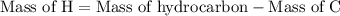 ...... (3)
...... (3)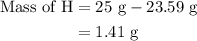
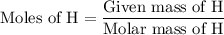 ...... (4)
...... (4)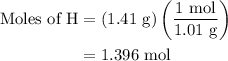
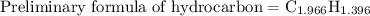

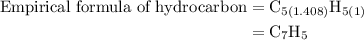
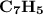 .
.


