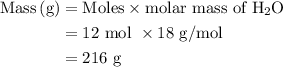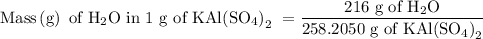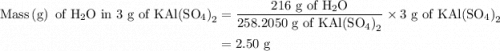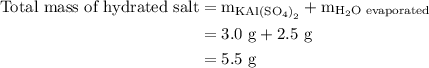Chemistry, 08.07.2019 14:30 christinapim
Suppose harry begins with the hydrate kal(so4)2·12h2o. after dehydration he finds that he is left with 3.0 g of the an-hydrate kal(so4)2. how many grams did he start with?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1

Chemistry, 23.06.2019 09:00
A2-kg bowling ball is 1 meter off the ground on a post when it falls. just before it reaches the ground,its traveling 4.4 m/s. assuming that there is no air resistant, which statement is true a. the initial potential energy is less then the final kinetic energy b. the mechanical energy is not conserved c. the mechanical energy is conserved d. the initial potential energy is greater than the final kinetic energy
Answers: 3

Chemistry, 23.06.2019 18:30
Achemist reacted 57.50 grams of sodium metal with an excess amount of chlorine gas. the chemical reaction that occurred is shown. na + cl2 ? nacl if the percentage yield of the reaction is 86%, what is the actual yield? show your work, including the use of stoichiometric calculations and conversion factors.
Answers: 1
You know the right answer?
Suppose harry begins with the hydrate kal(so4)2·12h2o. after dehydration he finds that he is left wi...
Questions

Business, 14.11.2020 21:00

Advanced Placement (AP), 14.11.2020 21:00

Chemistry, 14.11.2020 21:00



Computers and Technology, 14.11.2020 21:00





Mathematics, 14.11.2020 21:00


Mathematics, 14.11.2020 21:00


Mathematics, 14.11.2020 21:00

History, 14.11.2020 21:00



World Languages, 14.11.2020 21:00

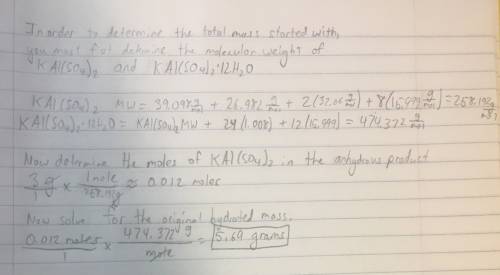
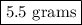 of
of 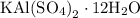 hydrated salt.
hydrated salt.
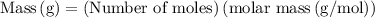
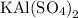 is 258.2050 g/mol.
is 258.2050 g/mol.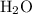 molecules is,
molecules is,