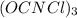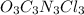Chemistry, 17.09.2019 11:30 faithb1466
The active ingredient in many types of household bleach has an empirical formula, ocncl. the molar mass is 232.41 grams per mole. what is the molecular formula of this compound? ocncl o2c2n2cl2 o3c3n3cl3 o4c4n4cl4

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2
You know the right answer?
The active ingredient in many types of household bleach has an empirical formula, ocncl. the molar m...
Questions




Mathematics, 19.09.2019 10:00



Social Studies, 19.09.2019 10:00


History, 19.09.2019 10:00




Mathematics, 19.09.2019 10:00

History, 19.09.2019 10:00

Mathematics, 19.09.2019 10:00



Social Studies, 19.09.2019 10:00

Health, 19.09.2019 10:00