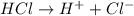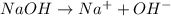1what do strong acids and strong bases have in common? a they both partially dissociate, with reverse reactions occurring. b they both dissociate completely, with little or no reverse reactions. c they both remain intact when placed in water, with no dissociation taking place. d they both dissociate completely, with reverse reactions constantly taking place.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
You know the right answer?
1what do strong acids and strong bases have in common? a they both partially dissociate, with reve...
Questions

Biology, 21.05.2021 19:40



Social Studies, 21.05.2021 19:40

Mathematics, 21.05.2021 19:40


Biology, 21.05.2021 19:40


Mathematics, 21.05.2021 19:40

Mathematics, 21.05.2021 19:40





Mathematics, 21.05.2021 19:40




Spanish, 21.05.2021 19:40

Mathematics, 21.05.2021 19:40