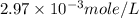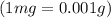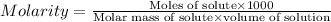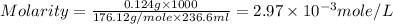Chemistry, 05.07.2019 00:40 ayoismeisalex
One cup of fresh orange juice contains 124 mg of ascorbic acid (vitamin c, c6h8o6). given that one cup = 236.6 ml, calculate the molarity of vitamin c in organic juice.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
One cup of fresh orange juice contains 124 mg of ascorbic acid (vitamin c, c6h8o6). given that one c...
Questions

Mathematics, 11.06.2020 00:57


English, 11.06.2020 00:57

Mathematics, 11.06.2020 00:57


Mathematics, 11.06.2020 00:57

Mathematics, 11.06.2020 00:57

Mathematics, 11.06.2020 00:57

Spanish, 11.06.2020 00:57


Mathematics, 11.06.2020 00:57


History, 11.06.2020 00:57



Mathematics, 11.06.2020 00:57


English, 11.06.2020 00:57

Mathematics, 11.06.2020 00:57

Social Studies, 11.06.2020 00:57