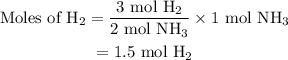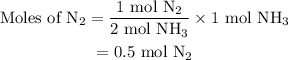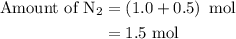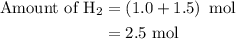Chemistry, 03.07.2019 16:30 campbell387
Amixture of n2(g) and h2(g) reacts in a closed container to form ammonia, nh3(g). the reaction ceases before either reactant has been totally consumed. at this stage 1.0 mol n2, 1.0 mol h2, and 1.0 mol nh3 are present. part a how many moles of n2 and h2 were present originally?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
Amixture of n2(g) and h2(g) reacts in a closed container to form ammonia, nh3(g). the reaction cease...
Questions




Mathematics, 25.06.2019 12:50


History, 25.06.2019 12:50







Mathematics, 25.06.2019 12:50




English, 25.06.2019 12:50



Mathematics, 25.06.2019 12:50

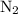 initially taken is
initially taken is 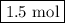 and the amount of
and the amount of 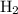 initially taken is
initially taken is 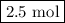 .
.
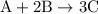
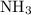 is as follows:
is as follows:
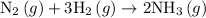
 .
.