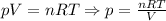Chemistry, 16.10.2019 05:30 carlosd21151
Consider three gases: ar, sf6, and cl2. if 50.0 grams of these gases are placed in each of three identical containers, which container will have the highest pressure? the volume and temperature of all three containers are the same.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
Consider three gases: ar, sf6, and cl2. if 50.0 grams of these gases are placed in each of three id...
Questions

Mathematics, 08.05.2021 14:00


Mathematics, 08.05.2021 14:00

English, 08.05.2021 14:00

Mathematics, 08.05.2021 14:00



Chemistry, 08.05.2021 14:00

Geography, 08.05.2021 14:00



Chemistry, 08.05.2021 14:00


Mathematics, 08.05.2021 14:00


Chemistry, 08.05.2021 14:00

Advanced Placement (AP), 08.05.2021 14:00