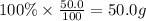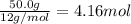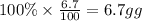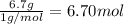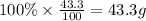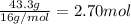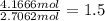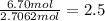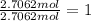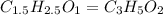Chemistry, 02.07.2019 18:40 pheonixhowls
What is the empirical formula of a compound that contains 50.0% carbon, 6.7% hydrogen, and 43.3% oxygen by mass? ch3o c3h5o2 c2h2o5 c3h205 c1h3o5

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2



Chemistry, 23.06.2019 13:20
Which nuclide is most likely to be radioactive and synthetic 24/12 mg237/93mg195/78mg230/84mg
Answers: 1
You know the right answer?
What is the empirical formula of a compound that contains 50.0% carbon, 6.7% hydrogen, and 43.3% oxy...
Questions



English, 09.02.2021 19:00

Arts, 09.02.2021 19:00

History, 09.02.2021 19:00







Mathematics, 09.02.2021 19:10






Mathematics, 09.02.2021 19:10


Mathematics, 09.02.2021 19:10

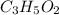 the empirical formula of a compound.
the empirical formula of a compound.