
Chemistry, 02.07.2019 14:50 connorhoran05
Calculate the resulting ph if 365 ml of 2.88 m hno3 is mixed with 335 ml of 1.10 m ca(oh)2 solution. be aware of stoichiometry!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
Calculate the resulting ph if 365 ml of 2.88 m hno3 is mixed with 335 ml of 1.10 m ca(oh)2 solution....
Questions


Biology, 22.06.2019 02:30


Mathematics, 22.06.2019 02:30

Social Studies, 22.06.2019 02:30

History, 22.06.2019 02:30














Mathematics, 22.06.2019 02:30

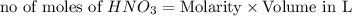
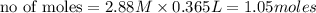
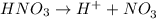
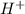 ions = 1.05
ions = 1.05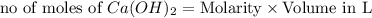
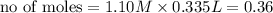
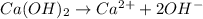
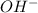 ions =
ions = 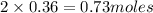
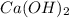 will neutralize 0.73 moles of
will neutralize 0.73 moles of 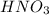 and (1.05-0.73)=0.32 moles of
and (1.05-0.73)=0.32 moles of 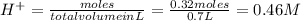
![pH=-\log[H^+]=-\log[0.46]=0.34](/tpl/images/0042/8951/36f67.png)


