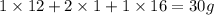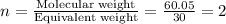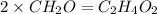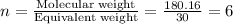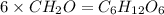The empirical formula for two compounds that have very different properties (one tastes very sour and the other very sweet) is ch2o. if the molar mass of compound a is 60.05 g/mol and compound b is 180.16 g/mol, what are the molecular formulas for these compounds, respectively?

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
The empirical formula for two compounds that have very different properties (one tastes very sour an...
Questions



Mathematics, 21.10.2021 07:30

English, 21.10.2021 07:30

History, 21.10.2021 07:30

Mathematics, 21.10.2021 07:30

Social Studies, 21.10.2021 07:30


Mathematics, 21.10.2021 07:30

Mathematics, 21.10.2021 07:30

Mathematics, 21.10.2021 07:30

Arts, 21.10.2021 07:30

Business, 21.10.2021 07:30

Mathematics, 21.10.2021 07:30


Mathematics, 21.10.2021 07:30

Mathematics, 21.10.2021 07:30


Physics, 21.10.2021 07:30

Mathematics, 21.10.2021 07:30

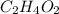
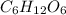
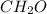 , empirical weight is =
, empirical weight is =