

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Pbco3 –> pbo+ co2. how many liters of carbon dioxide gas is produced from the decomposition of 32 grams of lead (ll) carbonate?
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
You know the right answer?
In the reaction mg(s) + 2hcl(aq) → h2(g) + mgcl2(aq), how many moles of hydrogen gas will be produce...
Questions

Computers and Technology, 20.11.2019 23:31

Biology, 20.11.2019 23:31





Chemistry, 20.11.2019 23:31

Business, 20.11.2019 23:31

Mathematics, 20.11.2019 23:31

Mathematics, 20.11.2019 23:31





English, 20.11.2019 23:31



Health, 20.11.2019 23:31


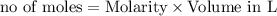
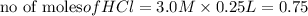
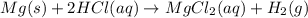
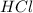 is a limiting reagent as it limits the formation of products and Mg is an excess reagent.
is a limiting reagent as it limits the formation of products and Mg is an excess reagent.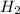
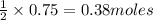 of
of 

