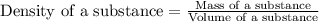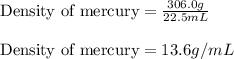Chemistry, 02.07.2019 13:10 oddoneshenchman
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml. the mercury used to fill the cylinder weighs 306.0 g. from this information, calculate the density of mercury.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml. the mercury used to fi...
Questions

Mathematics, 09.07.2021 19:10


Mathematics, 09.07.2021 19:10

Mathematics, 09.07.2021 19:10

English, 09.07.2021 19:10










History, 09.07.2021 19:10

Mathematics, 09.07.2021 19:10


Mathematics, 09.07.2021 19:10

Social Studies, 09.07.2021 19:20