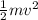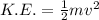Chemistry, 01.07.2019 09:20 oscaralan01
Which equation correctly relates kinetic energy mass and velocity

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Consider three unlabeled bottles, each contain small pieces of one of the following metals. - magnesium - sodium - silver the following reagents are used for identifying the metals. - pure water - a solution of 1.0 molar hcl - a solution of concentrated hno3 (a) which metal can be easily identified because it is much softer than the other two? describe a chemical test that distinguishes this metal from the other two, using only one of the reagents above. write a balanced chemical equation for the reaction that occurs. (b) one of the other two metals reacts readily with the hcl solution. identify the metal and write the balanced chemical equation for the reaction that occurs when this metal is added to the hcl solution. use the table of standard reduction potentials (attached) to account for the fact that this metal reacts with hcl while the other does not. (c) the one remaining metal reacts with the concentrated hno3 solution. write a balanced chemical equation for the reaction that occurs. (d) the solution obtained in (c) is diluted and a few drops of 1 m hcl is added. describe what would be observed. write a balanced chemical equation for the reaction that occurs.
Answers: 2

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
Which equation correctly relates kinetic energy mass and velocity...
Questions

Chemistry, 19.07.2019 16:50

Social Studies, 19.07.2019 16:50

History, 19.07.2019 16:50

Biology, 19.07.2019 16:50

Biology, 19.07.2019 16:50

Social Studies, 19.07.2019 16:50



History, 19.07.2019 16:50

Health, 19.07.2019 16:50





History, 19.07.2019 16:50




Social Studies, 19.07.2019 16:50

English, 19.07.2019 16:50