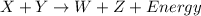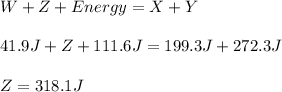Chemistry, 01.07.2019 02:40 osmarirodriguez2079
Reactant x contains 199.3 j of chemical energy. reactant y contains 272.3 j of chemical energy. product w contains 41.9 j of chemical energy. if the reaction loses 111.6 j of chemical energy as it proceeds, how much chemical energy must product z contain? 318.1 j 429.7 j 541.3 j 625.1 j

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Astudent made the lewis dot diagram of a compound shown. what is the error in the lewis dot diagram? a)an o atom should transfer all of its six electrons to mg because the formula is mgo b) both electrons of mg should be transferred to one o adam because the formula is mgo c) the electrons should be transferred from each o add him to capital mg because mg has fewer electrons d) the number of dots around mg should be four because it has to transfer two electrons to each o
Answers: 1


Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
Reactant x contains 199.3 j of chemical energy. reactant y contains 272.3 j of chemical energy. prod...
Questions









Mathematics, 04.04.2020 08:29



Health, 04.04.2020 08:29

Mathematics, 04.04.2020 08:29


Biology, 04.04.2020 08:29