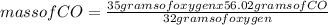When the amount of oxygen is limited, carbon and oxygen react to form carbon monoxide. how many grams of co can be formed from 35.0 grams of oxygen? 2c + o2 → 2co using 32.00 g/mole as the molecular mass of oxygen and 28.01 g/mole as the molecular mass of carbon monoxide, solve the above problem.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

You know the right answer?
When the amount of oxygen is limited, carbon and oxygen react to form carbon monoxide. how many gram...
Questions

English, 20.08.2021 19:40

Mathematics, 20.08.2021 19:40

Mathematics, 20.08.2021 19:40

Computers and Technology, 20.08.2021 19:40

English, 20.08.2021 19:40

Mathematics, 20.08.2021 19:40

English, 20.08.2021 19:40


Mathematics, 20.08.2021 19:40

History, 20.08.2021 19:40

Chemistry, 20.08.2021 19:40



Chemistry, 20.08.2021 19:50

Physics, 20.08.2021 19:50

Mathematics, 20.08.2021 19:50



Chemistry, 20.08.2021 19:50

Mathematics, 20.08.2021 19:50

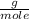 O₂: 32
O₂: 32