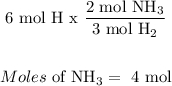Chemistry, 14.02.2023 14:00 itsmichaelhere1
What is the limiting reagent when 1.5 moles of nitrogen react with 6 moles of hydrogen? N2(g)+3H2(g)—> 2NH3(g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Type the correct answer in the box. spell all words correctly .what does biodiesel produce in higher amounts? biodiesel produces higher amounts
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


You know the right answer?
What is the limiting reagent when 1.5 moles of nitrogen react with 6 moles of hydrogen? N2(g)+3H2(g)...
Questions





Chemistry, 31.01.2021 22:10

Mathematics, 31.01.2021 22:10

Physics, 31.01.2021 22:10


World Languages, 31.01.2021 22:10


Mathematics, 31.01.2021 22:10




Mathematics, 31.01.2021 22:10


Mathematics, 31.01.2021 22:10

Social Studies, 31.01.2021 22:10