Calculate the molarity of 6.631 g NANO3 in 100.0 mL of solution
add stepss...

Chemistry, 01.05.2022 21:50 glstephens04
Calculate the molarity of 6.631 g NANO3 in 100.0 mL of solution
add stepss

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1

Chemistry, 23.06.2019 10:30
Can anyone explain 1. review your spectrometry data and use the known elements to identify the star's composition. which unknown elements make up this star? justify your element selections. 2. in parts i and ii of the lab, what happened to the electrons of each element to produce the different colors of light? explain your answers using important terms from the lesson and information provided in the laboratory. 3. stars composed of heavier (more massive) elements are often slightly older than stars made predominantly from hydrogen and helium. based on your data, is the newly discovered star a younger star? explain your answer.
Answers: 2
You know the right answer?
Questions

English, 26.09.2019 01:00


English, 26.09.2019 01:00









Chemistry, 26.09.2019 01:00


Mathematics, 26.09.2019 01:00






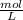
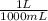 = 0.1000 L
= 0.1000 L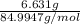 = 0.07802 mol
= 0.07802 mol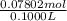 = 0.7802 M
= 0.7802 M

