Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
An occluded front moves over the farmland that has been experiencing drought conditions. what change in weather will this front likely bring? a. gray skies, but no rain b. an extended period of rain c. more dry air and sunny skies d. violent, short-lived thunderstorms
Answers: 3

Chemistry, 21.06.2019 16:30
In which direction will the following reaction go if the standard reduction potentials are 0.80 v for ag/ag+ and –0.44 v for fe/fe2+? ag+ + fe → ag + fe2+ a.)forward b.)the reaction cannot occur. c.) not enough information is given. d.) reverse
Answers: 1

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2
You know the right answer?
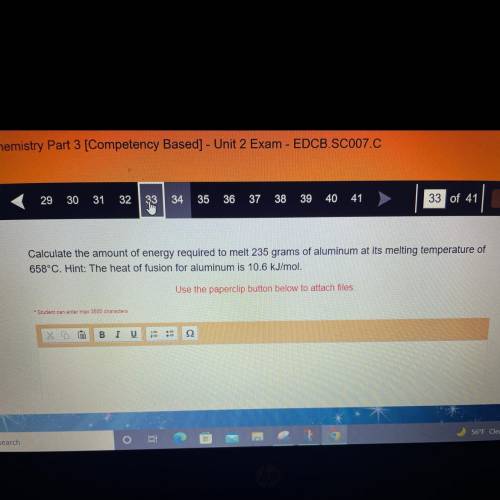
Calculate the amount of energy required to melt 235 grams of aluminum at its melting temperature of...
Questions

Mathematics, 13.01.2021 14:00

Geography, 13.01.2021 14:00

Engineering, 13.01.2021 14:00

Mathematics, 13.01.2021 14:00



Mathematics, 13.01.2021 14:00

History, 13.01.2021 14:00

Mathematics, 13.01.2021 14:00

Mathematics, 13.01.2021 14:00

Mathematics, 13.01.2021 14:00

Mathematics, 13.01.2021 14:00

Mathematics, 13.01.2021 14:00

Mathematics, 13.01.2021 14:00

Mathematics, 13.01.2021 14:00

English, 13.01.2021 14:00

Geography, 13.01.2021 14:00

Mathematics, 13.01.2021 14:00

Mathematics, 13.01.2021 14:00

Mathematics, 13.01.2021 14:00