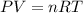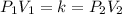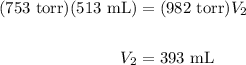Chemistry, 22.03.2022 03:50 mariaaking1413
What is the new volume of 513 mL of gas when the pressure changes from 753 torr to 982 torr? It is not necessary to convert torr to atm and assume a constant temperature.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Acar tire has a pressure of 2.38 atm at 15.2 c. if the pressure inside reached 4.08 atm, the tire will explode. how hot would the tire have to get for this to happen? report the temperature in degrees celsius.
Answers: 2

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 23.06.2019 01:00
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1

Chemistry, 23.06.2019 10:30
What is the difference between skimming and absorbing methods of the oil removal
Answers: 2
You know the right answer?
What is the new volume of 513 mL of gas when the pressure changes from 753 torr to 982 torr? It is n...
Questions

Mathematics, 05.10.2019 01:10



English, 05.10.2019 01:10









Social Studies, 05.10.2019 01:20



Mathematics, 05.10.2019 01:20


History, 05.10.2019 01:20

Health, 05.10.2019 01:20