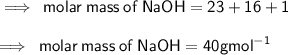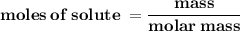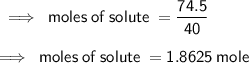Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3
You know the right answer?
What is the Molarity of a solution prepared by dissolving 74.5 g of NaOH in water to make 1.50 L of...
Questions

Spanish, 01.02.2021 09:50

Mathematics, 01.02.2021 09:50


Biology, 01.02.2021 09:50



Business, 01.02.2021 09:50

Biology, 01.02.2021 09:50


Mathematics, 01.02.2021 09:50




Mathematics, 01.02.2021 09:50

Mathematics, 01.02.2021 09:50

Computers and Technology, 01.02.2021 09:50

Chemistry, 01.02.2021 09:50

Medicine, 01.02.2021 09:50


Social Studies, 01.02.2021 14:00