
Chemistry, 17.03.2022 19:20 MegRasmussen31
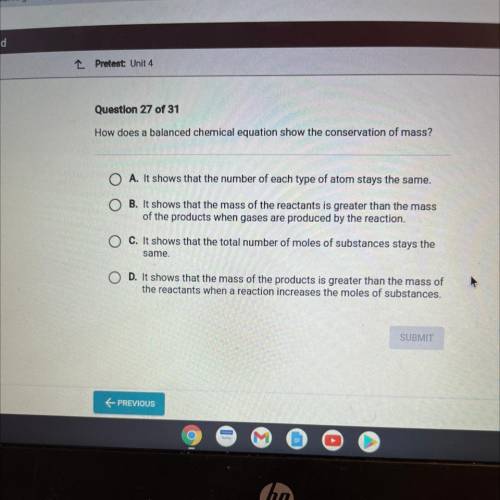
How does a balanced chemical equation show the conservation of mass?
O A. It shows that the number of each type of atom stays the same.
B. It shows that the mass of the reactants is greater than the mass
of the products when gases are produced by the reaction.
C. It shows that the total number of moles of substances stays the
same.
O D. It shows that the mass of the products is greater than the mass of
the reactants when a reaction increases the moles of substances.
SUBMIT

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2



Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
You know the right answer?
How does a balanced chemical equation show the conservation of mass?
O A. It shows that the number...
Questions



Chemistry, 18.12.2020 03:30

English, 18.12.2020 03:30


Biology, 18.12.2020 03:30



Mathematics, 18.12.2020 03:30




Computers and Technology, 18.12.2020 03:30

Spanish, 18.12.2020 03:30


History, 18.12.2020 03:30


History, 18.12.2020 03:30


Computers and Technology, 18.12.2020 03:30



