
Chemistry, 16.03.2022 20:00 kierafisher05
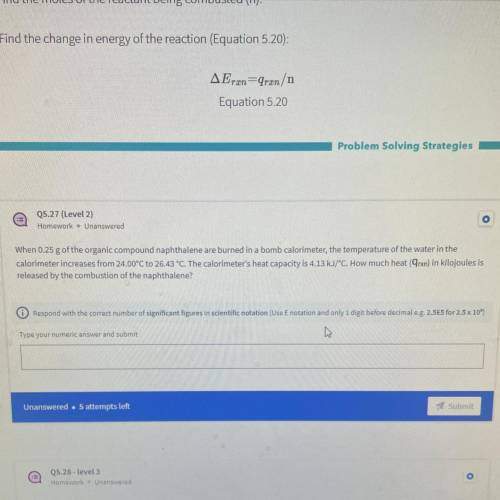
When 0.25 g of the organic compound naphthalene are burned in a bomb calorimeter, the temperature of the water in the
calorimeter increases from 24.00°C to 26.43 °C. The calorimeter's heat capacity is 4.13 kJ/°C. How much heat (9rxn) in kilojoules is
released by the combustion of the naphthalene?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2


Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
When 0.25 g of the organic compound naphthalene are burned in a bomb calorimeter, the temperature of...
Questions

History, 03.06.2021 20:40





Mathematics, 03.06.2021 20:40

Mathematics, 03.06.2021 20:40



Mathematics, 03.06.2021 20:40




Geography, 03.06.2021 20:40

Mathematics, 03.06.2021 20:40

Spanish, 03.06.2021 20:40

Mathematics, 03.06.2021 20:40


Mathematics, 03.06.2021 20:40

Social Studies, 03.06.2021 20:40



