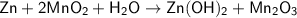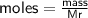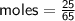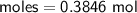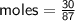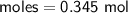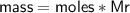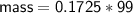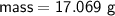Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
Zn + 2MnO2 + H2O → Zn(OH)2 + Mn2O3
Determine the limiting reactant if 25g of Zn and 30g of MnO2 ar...
Questions





Mathematics, 06.07.2019 01:30

Mathematics, 06.07.2019 01:30

Mathematics, 06.07.2019 01:30

Computers and Technology, 06.07.2019 01:30


Mathematics, 06.07.2019 01:30




Mathematics, 06.07.2019 01:30



Mathematics, 06.07.2019 01:30

Biology, 06.07.2019 01:30


Mathematics, 06.07.2019 01:30