
Chemistry, 12.03.2022 18:30 tahjaybenloss16
the slowest step in a reaction mechanism requires the collision represented above to occur. Which of the following most likely indicates how the addition of a solid catalyst could increase the rate of the reaction?
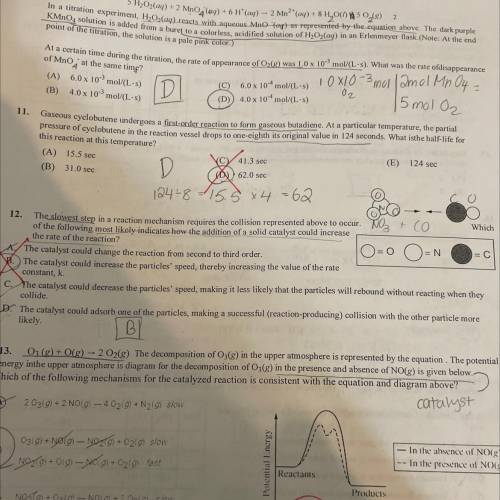

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2
You know the right answer?
the slowest step in a reaction mechanism requires the collision represented above to occur. Which of...
Questions

Mathematics, 15.12.2020 06:00


Mathematics, 15.12.2020 06:00

Mathematics, 15.12.2020 06:00




Mathematics, 15.12.2020 06:00

Mathematics, 15.12.2020 06:00

Mathematics, 15.12.2020 06:00




Mathematics, 15.12.2020 06:00



Biology, 15.12.2020 06:00

Mathematics, 15.12.2020 06:00

Biology, 15.12.2020 06:00

Health, 15.12.2020 06:00



