
Chemistry, 25.02.2022 07:10 Marshmallow6989
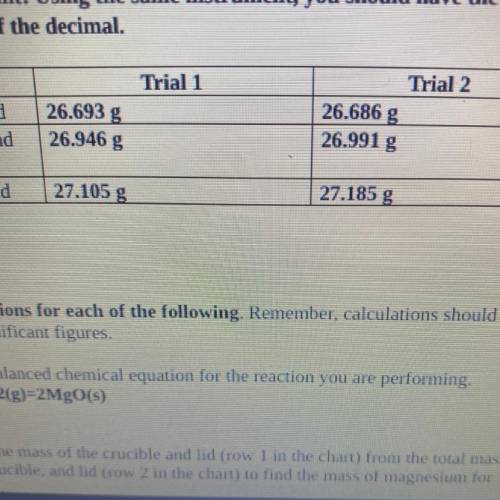
4. Magnesium is the limiting reactant in this experiment. Calculate the theoretical
yield of Mgo for each trial.
. Trial 1:
• Trial 2:
5. Determine the percent yield of Mgo for your experiment for each trial.
Trial 1:
• Trial 2:
6. Determine the average percent yield of Mgo for the two trials.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1
You know the right answer?
4. Magnesium is the limiting reactant in this experiment. Calculate the theoretical
yield of Mgo f...
Questions

Mathematics, 27.10.2020 01:40



Mathematics, 27.10.2020 01:40



Physics, 27.10.2020 01:40





Mathematics, 27.10.2020 01:40

Medicine, 27.10.2020 01:40

English, 27.10.2020 01:40

Mathematics, 27.10.2020 01:40

Medicine, 27.10.2020 01:40


Mathematics, 27.10.2020 01:40

Mathematics, 27.10.2020 01:40



