
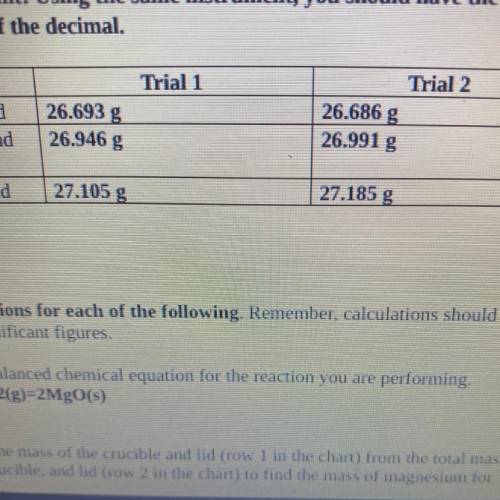
4. Magnesium is the limiting reactant in this experiment. Calculate the theoretical
yield of Mgo for each trial.
. Trial 1:
• Trial 2:
5. Determine the percent yield of Mgo for your experiment for each trial.
Trial 1:
• Trial 2:
6. Determine the average percent yield of Mgo for the two trials.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3


Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1
You know the right answer?
4. Magnesium is the limiting reactant in this experiment. Calculate the theoretical
yield of Mgo f...
Questions

Geography, 02.01.2022 14:00

Arts, 02.01.2022 14:00

English, 02.01.2022 14:00

World Languages, 02.01.2022 14:00


World Languages, 02.01.2022 14:00

Mathematics, 02.01.2022 14:00


History, 02.01.2022 14:00




Mathematics, 02.01.2022 14:00


Mathematics, 02.01.2022 14:00




Mathematics, 02.01.2022 14:00

Biology, 02.01.2022 14:00



