
Chemistry, 23.02.2022 14:00 BreBreDoeCCx
Write the rate law for the following reaction given that the order of A = 2, B = 1, and C = 0. A + B + C ———> D + E If the concentration of C is doubled, what will happen to the rate of the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1


Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1
You know the right answer?
Write the rate law for the following reaction given that the order of A = 2, B = 1, and C = 0. A + B...
Questions


Mathematics, 20.04.2021 06:50




Mathematics, 20.04.2021 06:50







Mathematics, 20.04.2021 06:50

Mathematics, 20.04.2021 06:50






![\displaystyle \text{Rate} & = k[A]^2[B]](/tpl/images/2667/2602/9cfbb.png)
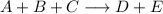
![\displaystyle \text{Rate} = k [A]^m[B]^n[C]^p](/tpl/images/2667/2602/aaf05.png)
![\displaystyle \begin{aligned} \text{Rate} & = k[A]^2[B][C]^0\\ \\ & = k[A]^2[B] \end{aligned}](/tpl/images/2667/2602/134df.png)


