
Chemistry, 22.02.2022 07:00 vapelordcarl69
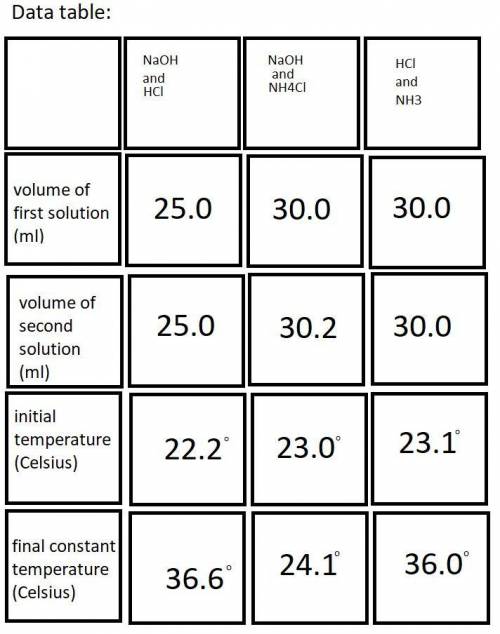
Do you have evidence from the lab that your calorimeter was not a perfect insulator? Assuming that heat was lost from the calorimeter, describe specifically how that would impact the data you recorded and then chase that change through the calculations to show how the error would impact the calculated enthalpy change for a particular reaction.
In the past, we have done this lab with roughly 50 mL each of each reactant solution, instead of 25 mL. Yet the Δ T determined experimentally were roughly the same. Explain why that is.
Each student will have slightly different volumes of reactants for each reaction. Yet the results obtained for ΔH1, ΔH2, and ΔH3 should be quite similar and would in fact be quite similar for any range of reactants combined within 25-50 mL, provided that each is accurately measured. Explain why this is the case.
A student misreads the volume of one of the solutions as 25.0 mL when in fact it was 35.0 mL. Step through the quantitative consequences of this error on the heat generated, the Δ T observed, the q calculated, and the Δ H calculated. (Clarification: Both solutions “should” be 25.0 mL or something close, and one of the volumes is accidentally 35.0 mL)
A student pours the reaction solutions into a still wet Styrofoam cup. Step through the quantitative consequences of this error on the heat generated, the Δ T observed, the q calculated, and the Δ H calculated.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 13:00
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
You know the right answer?
Do you have evidence from the lab that your calorimeter was not a perfect insulator? Assuming that h...
Questions

Mathematics, 30.09.2019 22:00


Computers and Technology, 30.09.2019 22:00


Biology, 30.09.2019 22:00

Biology, 30.09.2019 22:00







English, 30.09.2019 22:00





Computers and Technology, 30.09.2019 22:00

Mathematics, 30.09.2019 22:00




