
Chemistry, 21.02.2022 07:10 nicolehathaway1012
Bismuth oxide reacts with carbon to form bismuth metal:
Bi2O3(s) + 3C(s) → 2Bi(s) + 3CO(g)
When 447 g of Bi2O3 reacts with excess carbon,
(a) how many moles of Bi form?
(b) how many grams of CO form?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2
You know the right answer?
Bismuth oxide reacts with carbon to form bismuth metal:
Bi2O3(s) + 3C(s) → 2Bi(s) + 3CO(g)
Questions


History, 25.08.2019 04:00




Physics, 25.08.2019 04:00

Chemistry, 25.08.2019 04:00

Mathematics, 25.08.2019 04:00

Chemistry, 25.08.2019 04:00


Mathematics, 25.08.2019 04:00




History, 25.08.2019 04:00



History, 25.08.2019 04:00


Social Studies, 25.08.2019 04:00

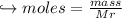
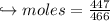
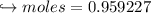
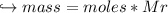
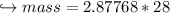
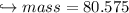 g
g

