Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asmall amount of a solid is added to water. the observation made after fifteen minutes is shown in the figure. which of these solids has been probably added to water? a) oil b) sand c) sugar d) wood chips
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2


Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
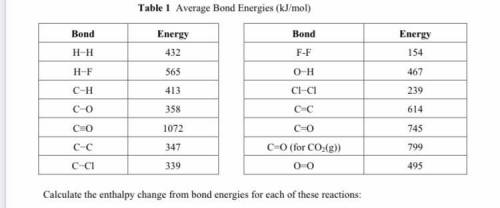
Calculate the enthalpy change from bond energies for each of these reactions:
CO(g) + 2H2(g) → CH3...
Questions

Social Studies, 16.03.2022 21:40


Biology, 16.03.2022 21:40

Chemistry, 16.03.2022 21:40



History, 16.03.2022 21:50

Mathematics, 16.03.2022 21:50


Mathematics, 16.03.2022 21:50






Business, 16.03.2022 21:50




Mathematics, 16.03.2022 22:00