
Chemistry, 19.02.2022 08:20 destinymitchell966
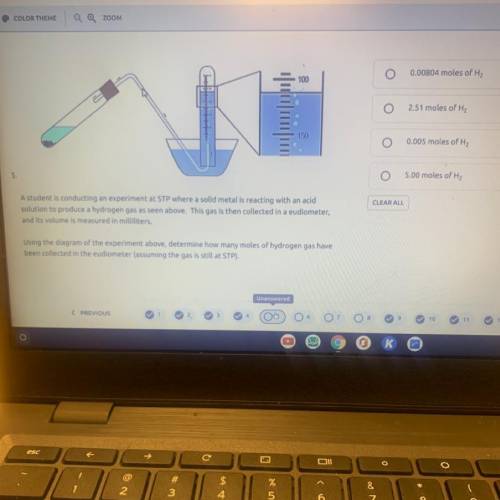
A student is conducting an experiment at STP where a solid metal is reacting with an acid solution to produce a hydrogen gas as seen above. This gas is then collected in a eudiometer, and its volume is measured in milliliters. Using the diagram of the experiment above, determine how many moles of hydrogen gas have been collected in the

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2


Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1
You know the right answer?
A student is conducting an experiment at STP where a solid metal is reacting with an acid solution t...
Questions

English, 15.01.2020 06:31


Biology, 15.01.2020 06:31

Mathematics, 15.01.2020 06:31

Mathematics, 15.01.2020 06:31




Advanced Placement (AP), 15.01.2020 06:31

Social Studies, 15.01.2020 06:31

Mathematics, 15.01.2020 06:31


Health, 15.01.2020 06:31


Mathematics, 15.01.2020 06:31



Social Studies, 15.01.2020 06:31

Social Studies, 15.01.2020 06:31

Advanced Placement (AP), 15.01.2020 06:31



